Dosepak® Medication Compliance Packaging
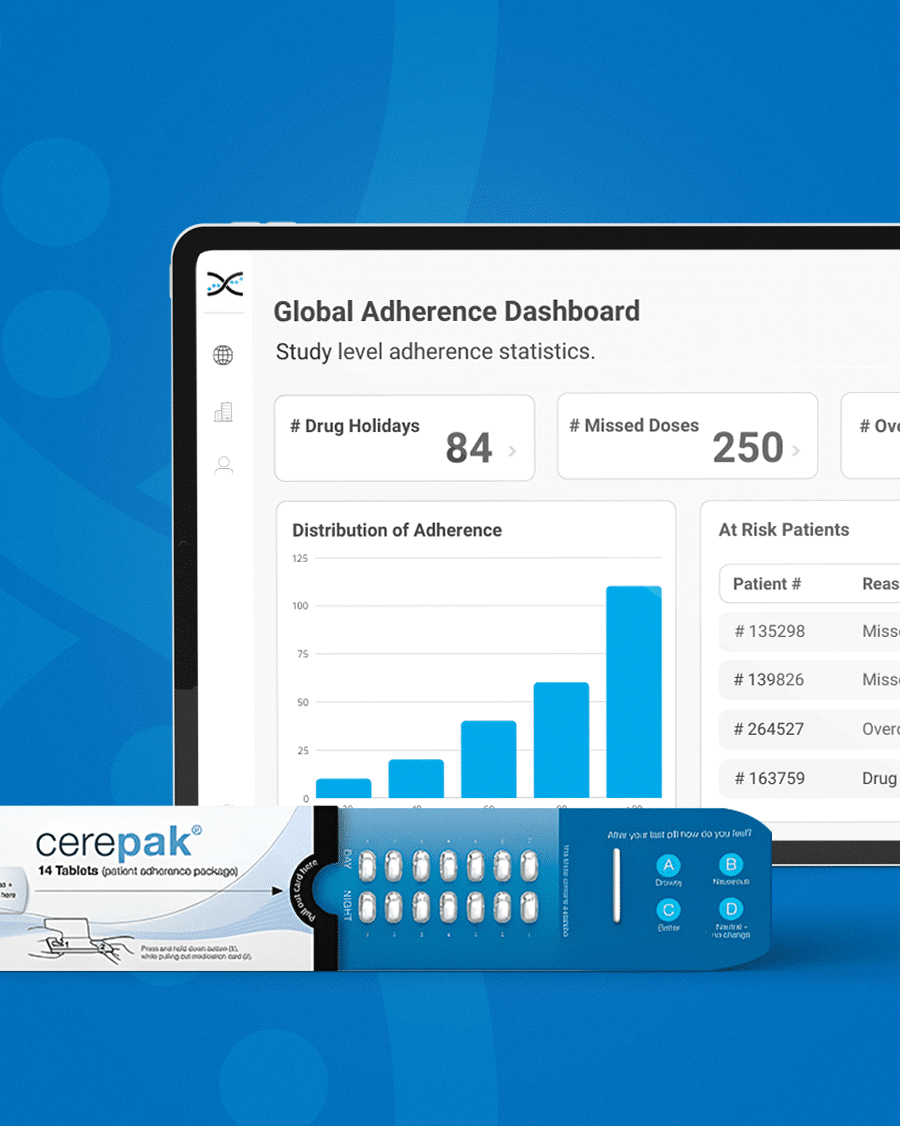
Introducing Electronic Dosepak (EDP), an innovative medication compliance packaging solution developed by Westrock. EDP features a reusable electronic e-module powered by AARDEX Group's MEMS® technology to monitor and record each package opening and closing date and time. EDP technology promotes treatment plan compliance, and with its seamless integration with our medication adherence software, researchers can benefit from valuable insights. By capturing this information, the solution assists in identifying patterns of medication usage, allowing for more personalized care for patients

ELECTRONIC DOSEPAK (EDP)
Improve Medication Compliance with EDP
The Electronic Dosepak goes beyond the conventional medication compliance packaging solution by harnessing digital capabilities to transform treatment compliance and significantly impact research globally.
How does Dosepak® work?
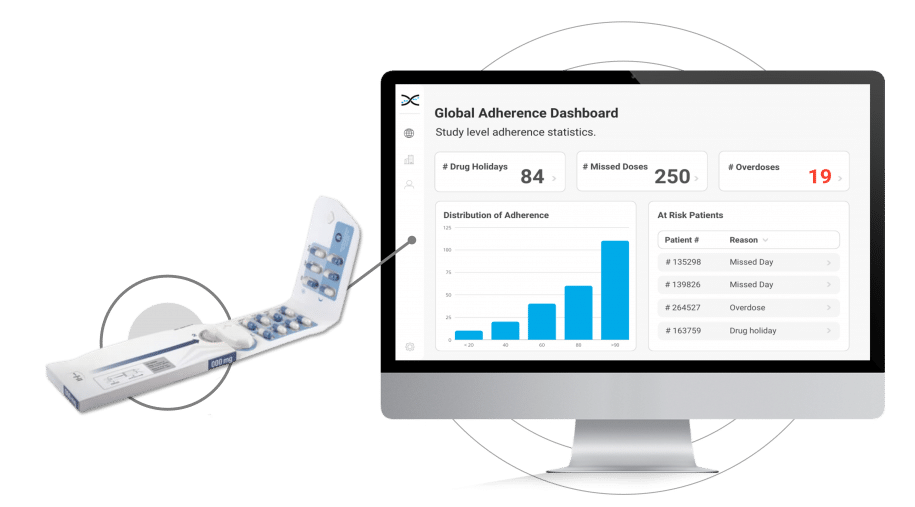
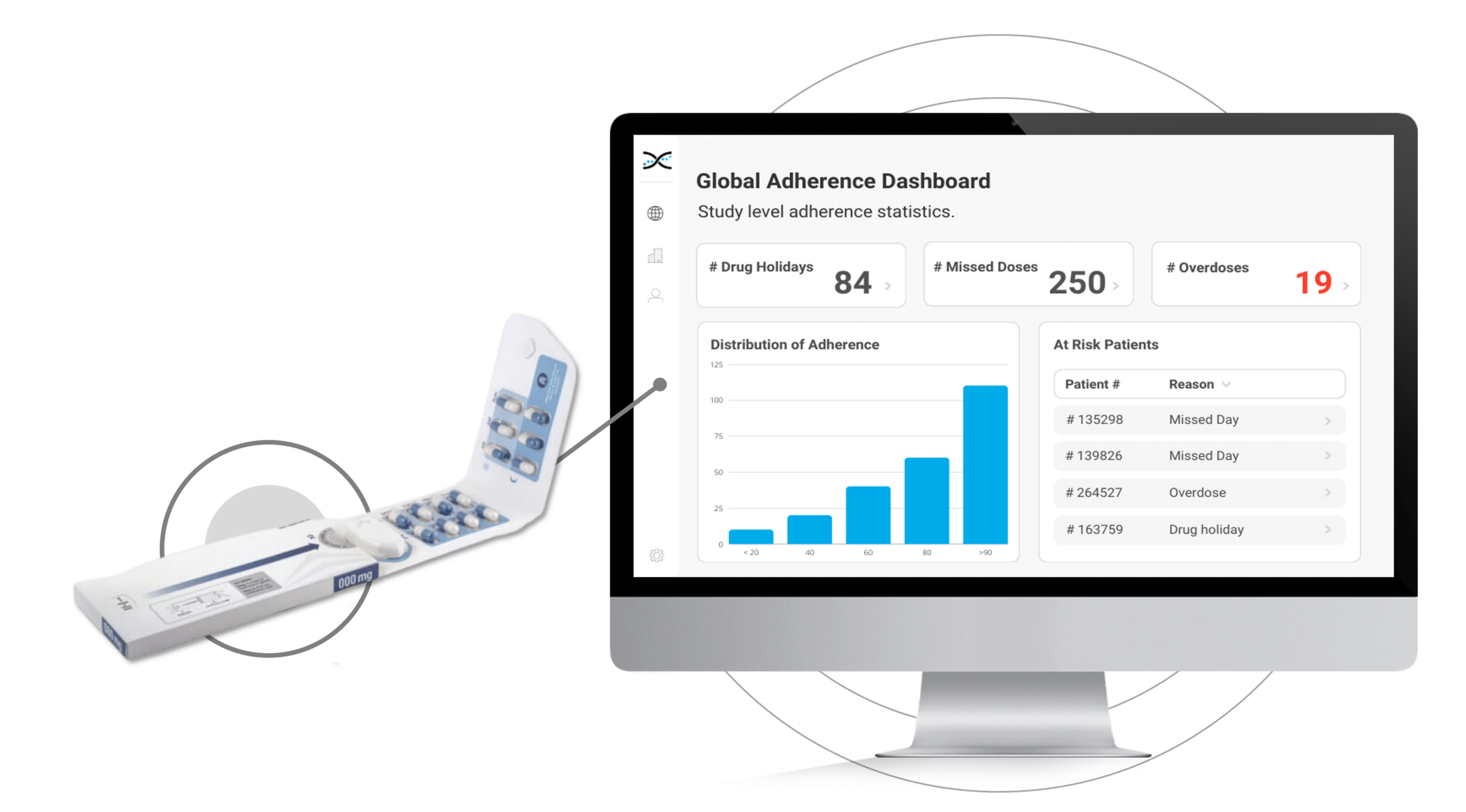
Its reusable electronic module diligently logs the date and time each time the Dosepak is opened and closed, offering unprecedented accuracy and precision.However, this is only the beginning. Dosing data from the Electronic Dosepak is effortlessly integrated with our Medication Adherence Software, MEMS AS®, where sophisticated statistical algorithms analyze the data and generate a series of automated dashboards for investigators to assess treatment adherence at the study, site, and patient level. These user-friendly dashboards deliver crucial insights into patients' medication-taking behaviors, allowing healthcare researchers to detect problematic compliance patterns and make well-informed decisions to enhance patient outcomes.
What are the benefits of Dosepak® Medication Compliance Packaging?
By utilizing the Electronic Dosepak and MEMS AS, researchers can have confidence in accessing precise and dependable data on medication compliance. This results in a more efficient approach to medication management, empowering researchers to provide tailored care and assistance to their patients. Opt for the Electronic Dosepak to experience the advantages of state-of-the-art technology and patient-centric design in medication compliance packaging.Features of Dosepak® Medication Compliance Packaging Integrated with MEMS AS®
FEATURES
Real-Time Treatment Compliance Monitoring.
The Electronic Dosepak's seamless integration with our proprietary Medication Adherence Software, MEMS AS, provides clinical researchers with a comprehensive solution for monitoring treatment compliance in real-time. This user-friendly software features a variety of pre-built dashboards and ranked lists, enabling researchers to effortlessly track adherence patterns at the participant, site, and study levels. Access to real-time compliance insights is invaluable for clinical trials. It allows researchers to proactively identify adherence issues and implement suitable measures to ensure participants maintain their medication regimens.
- Fully Scalable
- ISO27001 Certified Datacenter
- HIPAA & GDPR Compliant
- IRT, EDC & DCT Integration

FEATURES
Unparalleled Accuracy
and Reliability.
Our innovative approach to medication compliance monitoring stands out from conventional methods such as pill count, self-report, and PK sampling, offering exceptional accuracy and a range of distinct advantages. Studies have demonstrated that treatment compliance packaging achieves an impressive 97% accuracy rate in capturing and recording participant medication events. In contrast, PK sampling reaches only 70%, pill count 60%, and self-report a modest 27%. By leveraging our cutting-edge adherence solutions, researchers obtain highly accurate data that contributes to safety and efficacy assessments, empowering them to take action to bolster patient safety. The superiority of our approach over traditional methods makes it an essential tool in modern clinical research, ensuring both precision and reliability in medication monitoring.
- Participant Acceptability High
- User-Friendly Solutions

FEATURES
Flexible Compliance Packaging.
Dosepak transcends the role of typical compliance packaging. Its highly customizable design features a calendarized blister format, which allows clinical trial sponsors to incorporate custom branding, informative messages, and dosing guidelines on the outer packaging. This enhances the patient experience, fosters brand awareness, and aids in medication compliance tracking. Don't compromise on quality – choose Dosepak to experience the benefits of cutting-edge technology and a patient-oriented approach first hand.
- Child-Resistant
- 18 Month Battery Life
- CE-Marked Solution

FEATURES
Participant-Centric Solution.
Addressing obstacles to compliance in clinical trials is crucial for enhancing participant outcomes and preserving research validity. We understand the significance of user-friendly solutions that minimize the burden on participants, only necessitating that they take their medication according to the prescription. Moreover, Electronic Dosepak integrates with MEMS® Mobile, a user-friendly app that enables participants to set medication reminders, assisting them in maintaining their dosing schedules. Our patient-focused approach to medication adherence aims to empower participants and facilitate improved outcomes. By incorporating Electronic Dosepak with MEMS Mobile, we bring this vision to life, offering a seamless and effective solution for medication management in clinical trials.
- User-Friendly App
- IoS & Android Compatible
- Available in 25 Languages
- 20K+ Users in 30 Countries

FEATURES
Approach Backed by the FDA.
In the FDA's guidance, "Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products," there is a resounding emphasis on the importance of prioritizing medication adherence in the research industry. To achieve this, the FDA endorses the use of medication compliance packaging to promote patients' adherence to their prescribed regimens. By harnessing the power of technology, researchers can proactively address medication management, ultimately providing more tailored and efficacious care.
- ICH GCP Compliant
- FDA 21 CFR part 11 Compliant

OUR CLIENTS
The Go-To Solution for Pharma Companies
Some of the world's leading pharmaceutical companies have embraced our medication adherence solutions. From global giants to niche players, these organizations have recognized the value of our innovative solutions for enhancing medication adherence, reducing costs, and improving patient outcomes. It's an honor to partner with these remarkable brands, and we're proud to contribute to their efforts in advancing healthcare.
Medication Adherence Software →
Learn about our industry-leading adherence software for trials.

Medication Adherence Packaging →
Discover our range of medication adherence packaging.

Discover our range of medication adherence devices.
Got Questions?
Connect with an adherence expert.
Frequently Asked Questions
Treatment compliance is a vital yet often overlooked aspect of successful research. That's why we've gone the extra mile to gather and organize the most frequently asked questions about this critical topic. Our goal is to empower researchers and patients alike with the knowledge they need to ensure medication adherence is never a hurdle to progress. So, without further ado, here are the answers you've been looking for!
Treatment compliance, adherence, and concordance are all terms used to describe a patient’s behavior concerning following a prescribed treatment regimen, but they are not entirely interchangeable.
Treatment compliance generally refers to whether or not a patient follows the prescribed treatment regimen as directed by their healthcare provider. Compliance typically implies a more authoritarian approach to treatment, with the healthcare provider having the primary responsibility for making decisions and the patient following those decisions.
Adherence, on the other hand, is a broader term that encompasses the concept of compliance but also considers the patient’s personal beliefs and motivations. Adherence involves actively choosing to follow the treatment regimen based on an understanding of the benefits and risks of the treatment, as well as the patient’s personal values and preferences.
Concordance is a newer term emphasizing the importance of shared decision-making between the patient and healthcare provider. Concordance involves working collaboratively with the patient to reach an agreement on the best course of treatment, taking into account the patient’s values, preferences, and circumstances. This approach recognizes that treatment is most effective when the patient feels involved in the decision-making process and has a sense of ownership over their treatment plan.
In summary, while these terms are related to each other, they have distinct meanings that reflect different approaches to the patient-provider relationship and emphasize different aspects of the patient’s role in treatment.
Treatment compliance is essential for the effective management of many medical conditions. Following prescribed treatment regimens make patients more likely to achieve positive treatment outcomes, such as improved symptom control, disease management, and better overall health.
In clinical trials, treatment compliance is crucial because it directly affects the validity and reliability of the study’s results. If participants do not follow the prescribed treatment regimen, it can be challenging to determine the true efficacy and safety of the medicine being tested. Poor adherence can introduce bias to the study’s results and lead to variability in outcomes, which can impact treatment decisions for patients in the future.
Moreover, non-compliance can lead to disease progression, complications, and treatment failure and may result in the need for additional healthcare interventions, including hospitalization, further testing, and surgery. This can increase healthcare costs and prolong recovery time for patients.
Various factors can influence a patient’s compliance with treatment plans, such as the complexity of the treatment regimen, the patient’s beliefs, and attitudes, the availability of social support, access to healthcare, health literacy, cognitive or physical impairment, and mental health conditions. These factors can differ from one patient to another and affect adherence differently depending on the individual’s situation. Healthcare providers should consider these factors when designing treatment plans to promote compliance and optimize treatment outcomes.
WEBINAR WITH MERCK & BIOGEN
Mitigating the Risk of Poor Adherence in Trials
Watch this live recording with adherence experts from Merck & Biogen to learn about their approach to mitigating the risk of poor adherence in trials.


Collaborating for Safer, More Efficient Trials.
By combining technology and partnerships, we are revolutionizing how medication adherence is monitored in clinical trials. Our unique adherence ecosystem brings together leading medication adherence packaging and devices and DCT, IRT, and EDC vendors, CROs, and CMOs to drive innovation.
