Study power is the Holy Grail of clinical trials, setting drug developers on the quickest path to market – but non-adherence causes costly detours.
When participants do not take their medication as specified in the study protocol, it can result in underestimated drug efficacy, delay the approval of investigational products, and contribute to rising costs.
Without mitigation, then, the problem of medication non-adherence can have a significant impact on clinical trial return on investment (ROI).
The Scale of the Problem
A staggering 80% of clinical studies fail to finish recruitment on time, and 20% of these are delayed for at least six months[1] – and every single day of delay to market can cost between $600,000 and $8million in lost revenue.[2]
Taking control of adherence is one way to recoup costs and improve ROI. Studies show that each patient involved in a Phase III trial is responsible for an average of $42,000 in costs,[3] yet almost a third, 30%, are non-adherent by day 100.[4]
The problem isn’t confined to late-stage studies. Across all clinical trials, 50% of participants admit to not adhering to the dosing regimen set out in the protocol.[5]
Such protocol deviations usually go undetected by traditional, non-digital methods of measurement, such as pill counts, blood sampling, and self-reporting by trial participants.
The Impact on Study Power
Study power is an essential component of clinical trial design, but non-adherence to medication during studies can thwart even the best-laid plans.
The higher the study power, the higher the probability of detecting a drug’s true effect. Higher statistical power increases the chances of success, enables more informed go/no go decisions, and shortens the time to market.
It depends on three factors, namely the effect size of the drug itself, the variability in drug response, and the sample size. The sample size must be large enough to show the maximum effect size with the lowest variability.
But when subjects do not take their medication as directed, it decreases effect size and increases variability, and, therefore, drains study power. The exponential relationship between non-adherence and sample size means that any decrease in adherence must be met with an increase in study participants if power is to be maintained.
Non-adherence, then, has a direct effect on the cost and duration of clinical trials, reducing ROI.
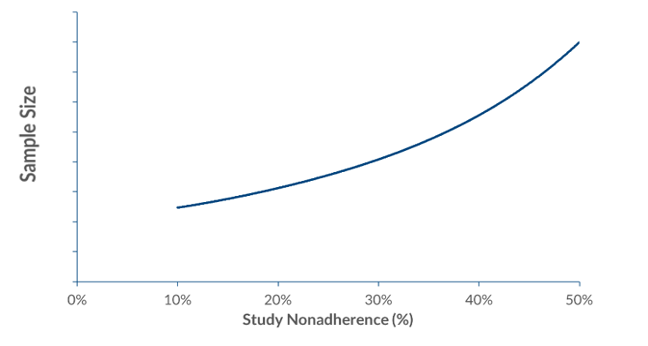
Figure: typical relation between study nonadherence and sample size
Mitigation Strategies
There are three ways to mitigate the impact of non-adherence on clinical trial ROI: increase the sample size, select a higher dose, or manage adherence.
The first of these is an expensive option which can have a direct impact on recruitment times and delay time to market.[6]
Studies have shown that a 20% non-adherence rate among subjects requires a 50% increase in a sample size to maintain equivalent statistical power.[7] This kind of expansion, at an average cost of £42,000 per subject for a Phase III study,3 is not only expensive but also adds to the complexity of recruitment and study management.
Selecting a higher dose than an adherent patient would need to demonstrate an effect is self-evidently a risky approach with potential safety implications.[8]
Rather counterproductively, inappropriate dose setting can cause side effects that actually result in worse compliance, hence it being the number one reason for failed or delayed FDA approval. Even when a drug developed in this way does make it to market, it is often at a reduced dose than that seen in trials.
Both strategies are not only a waste of time, money, and patient experience but they compromise estimates of the benefits and risks of a medicine.
Managing Medication Adherence
Rather than mitigating undetected non-adherence by attempting to enroll more subjects or risk safety with increased dosing, many sponsors are choosing digital medication adherence management.
Studies have shown that smart package monitoring is 97% accurate, compared to 60% for pill count, 50% for healthcare professional rating, and just 27% for self-report. It is a continuous method that puts no extra burden on participants, is available to all subjects, and automatically feeds information back to the study team so they can take action.
Such innovations, which can cost as little as $1 a day, maximize exposure through feedback and interventions and provide adherence-informed analytics that compliment intention-to-treat (ITT) analytics. By doing so they can improve adherence to medication during clinical trials by up to 50%.[9]
Additionally, these systems improve data quality and integrity, support evidence-based risk mitigation strategies, and ensure compliance with FDA and ICH guidelines.
Crucially, they give drug developers an insight into how their products are used “in real life”, and provide behavioral data that can be utilized in the design of successful marketing strategies.
More and more organizations are finding that this approach can help extract every drop of power from a study, thus reducing time to market, and increasing ROI. AARDEX’s solutions alone have been used in more than 1million study participants, including 200-plus Phase II/III trials.
Medication Adherence Management and ROI
A typical Phase III study will last for one year and cover 50 sites; it will include 850 participants on a four-month follow-up – and will experience 20% non-adherence.8
An additional 425 subjects would be needed to compensate for the associated drop in study power through increasing sample size.
Aside from the direct costs of more than $18million, such an undertaking would delay the conclusion of the trial, lengthening time to commercialization and resulting in an important loss of revenue.
But market-leading adherence management systems enable study managers to preserve study power by measuring and managing medication adherence throughout a trial. They can cost as little as $1 a day, meaning direct costs are significantly lower than increasing sample size, and they have no delaying impact on study duration or time to market.
It means that for less than 1% of trial cost, medication adherence management can stop the study power drain, unlock the full power of clinical data, and maximize ROI.
AARDEX is the market leader in measuring and managing medication adherence in clinical trials.
[1] Considerations For Improving Patient Recruitment Into Clinical Trials. (n.d.). https://www.clinicalleader.com/doc/considerations-for-improving-patient-0001#:~:text=Nearly%2080%25%20of%20all%20clinical,enrollment%20for%20a%20given%20trial
[2] Hargreaves, B. Clinical trials and their patients: The rising costs and how to stem the loss. (2016) http://www.pharmafile.com/news/511225/clinical-trials-and-their-patients-rising-costs-and-how-stem-loss
[3] Examination of Clinical Trial Costs and Barriers for Drug Development. (2014). https://aspe.hhs.gov/system/files/pdf/77166/rpt_erg.pdf
[4] Blaschke, T., et al. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. (2011) https://pubmed.ncbi.nlm.nih.gov/21942628/
[5] Eliasson L,. et al. How the EMERGE guideline on medication adherence can improve the quality of clinical trials. (2020). https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bcp.14240
[6] Breckenridge A, et al. Poor medication adherence in clinical trials: consequences and solutions. (2017). https://pubmed.ncbi.nlm.nih.gov/28154411/
[7] Alsumidaie, M. Non-Adherence: A Direct Influence on Clinical Trial Duration and Cost. Applied Clinical Trials. (2017) http://www.appliedclinicaltrialsonline.com/non-adherence-direct-influence-clinical-trial-duration-and-cost
[8] AVG 2014 phase III https://clinicaltrials.gov/
[9] Demonceau, J., et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. (2013). https://pubmed.ncbi.nlm.nih.gov/23588595/



